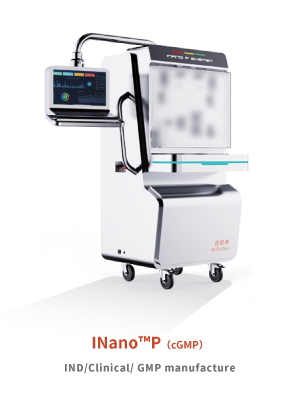
INano ™ P(IND/Clinical/ GMP manufacture)
INano ™ P(IND/Clinical/ GMP manufacture)
INano ™ P(IND/Clinical/ GMP manufacture)
- Detail
- Parameters
IND/Clinical/ GMP manufacture
>48L/hr
Features∶
1、Unique process technology,waste < 20mL
2、Highly automated, capable of automatic exhaust and automatic switching waste liquids.
3、Multiple modules are available (flow sensor,On-line DLS)
4、Complete biocompatibility research data on contact materials
5、Production capacity: from 100ml to several tens of liters.
6、Provide equipment GMP validation activities and documentation support
7、Equipments complies with cGMP production requirements and FDA 21CFR Part 11 requirements